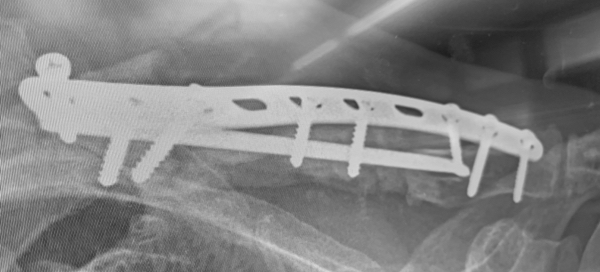
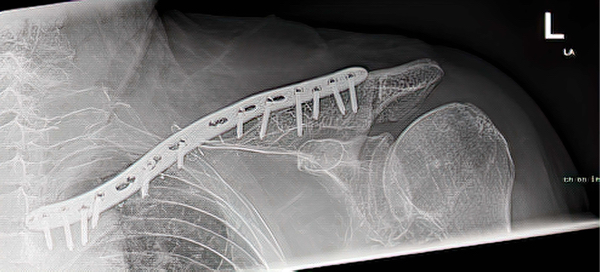
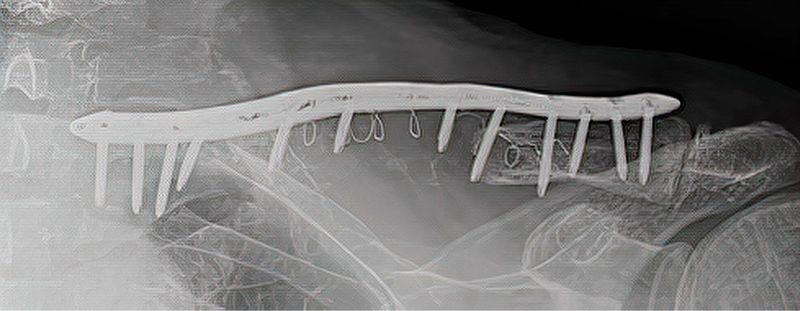
An 83 year old male experienced a fracture of the left clavicle associated with metastatic disease. During a previous operative procedure large parts of the clavicle were removed and the clavicle was appropriately shortened. The residual gap in the clavicle was supported with a Titanium Elastic Nail (TENs) that was was inserted into the proximal and distal ends of the clavicle and sealed in place with bone cement. In addition to the TENs nail, a plate spanned the gap with the ends of the plate affixed to the ends of the construct with screws. The result was unstable and very painful for the patient.
During a revision procedure, the plate and TENs nail were removed and an IlluminOss Implant was delivered from the lateral side,bridged the gap and inserted the implant into the medial segment. The spanning implant connected the two remaining fragments of the clavicle. The implant was filled with monomer and cured with visible light. After curing, the construct was further stabilized with the placement of a longer plate and screwed in place. Post procedure the patient has satisfactory stability and is pain-free.