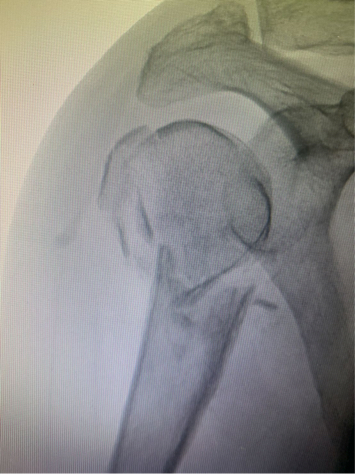
Multiple approaches were used to address this fracture in a 60-year-old male, smoker, functional alcoholic patient with osteoporosis. Initially, nonoperative treatment was attempted; however, after two weeks, his displacement and pain levels had risen to the point that operative fixation was performed.
A direct lateral approach was made, and identification of the axillary nerve achieved. The head segment of the humerus was controlled through the use of #2 FiberWires in the supraspinatus and subscapularis. Then a modification of the parachute technique was utilized to create a valgus impacted pattern by crossing the fibers under the axillary nerve and affixing them with two 3.5mm swivel-lock anchors.
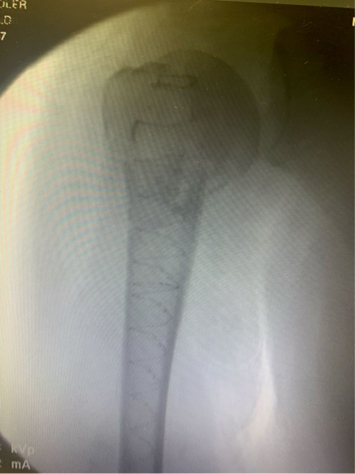
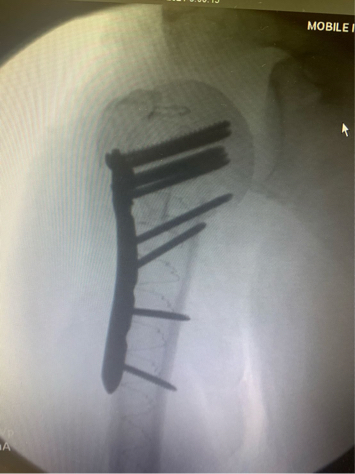
Once preliminary fixation was achieved, implant purchase was enhanced by placing a 22/13mm x 160mm tapered IlluminOss implant in the canal, filling it with monomer and curing it with blue light. After curing, the entire construct was spanned with a lateral-based proximal humerus plate with all screws having improved purchase and decreased working length to prevent toggle and loosening.
Dynamic fluoro evaluation noted solid movement. While weight-bearing was restricted, the patient was allowed immediate active and passive motion.