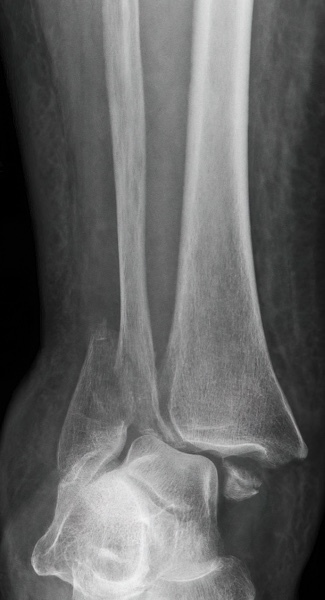
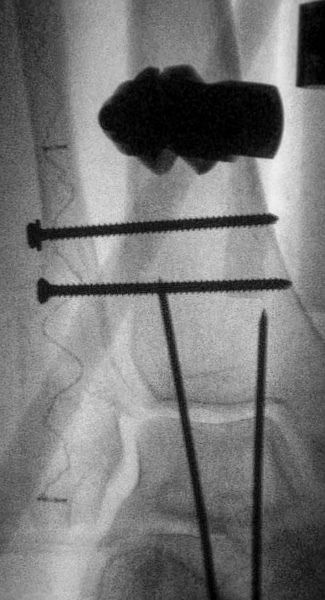
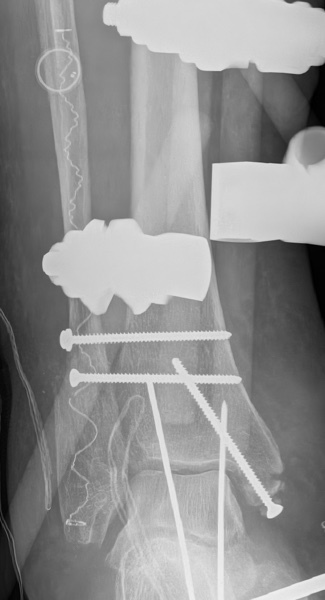
IlluminOss was used to treat a 79-year-old female patient who suffered a fall at home and sustained a trimalleolar fracture with ankle dislocation. The patient suffers from co-morbidities including osteoporosis, compromised soft tissue in the lower extremity, and metastatic breast cancer. The patient was treated initially with a reduction of the ankle joint and application of an external fixator. The fibula was fixed percutaneously by using an IlluminOss 9mm x 160mm implant and 2 syndesmotic screws, a medial malleolus screw, and Steinman pins for ankle stability. External fixator and Steinmann pin removal planned for 6 weeks post-op.
Download PDF | 900631-B