86-year-old female, slipped and fell, suffering a trimalleolar fracture. The patient had significant multiple comorbidities, including poor soft tissue and significantly osteoporotic bone. The fibula was percutaneously stabilized using an IlluminOss 9mm x 160mm implant, followed by the application of a minimally invasively placed distal lateral fibula plate. The medial malleolus was treated with a plate and screws. A screw was placed through the fibular fixation plate and the IlluminOss implant into the tibia. Postoperatively, the patient was allowed to weight bear as tolerated. At 6-weeks, the screw going from the fibular plate into the tibia was removed.
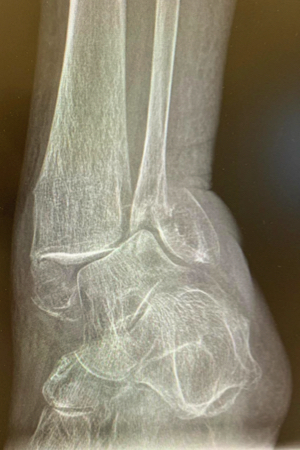
Pre-Op
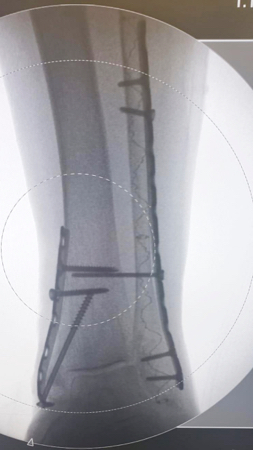
Post-Op
Download PDF | 900632_B