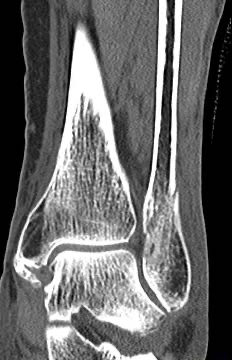
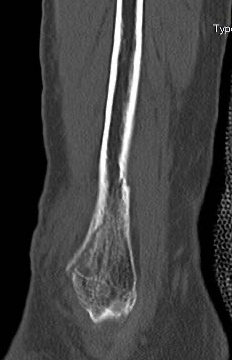
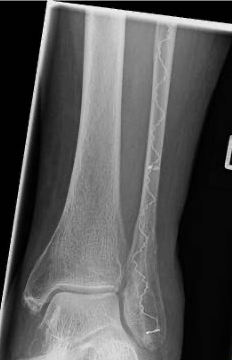
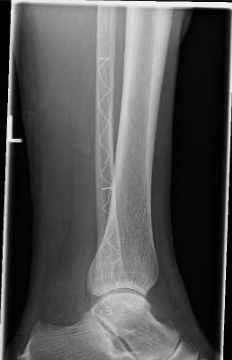
An IlluminOss implant was used in the treatment of a Weber B fracture on an 81-year-old female patient suffering a Weber B fracture of the left fibula after a fall. Using fluoroscopy, a sharp clamp was positioned percutaneously in order to achieve and maintain reduction of the fracture. A small incision was made at the distal lateral malleolus and a pathway was created with a cannulated 4.0mm awl to allow the canal to be prepared with flexible burrs of 5.5mm and 6.5mm diameter delivered over a 1.2mm guide wire. A 9mm x 160mm implant with a 4.5mm sheath was introduced into the canal and infused with monomer expanding the implant and filling the canal. Positioning of the implant and reduction of fracture was controlled using fluoroscopy. The implant was polymerized using visible blue light from the IlluminOss lightbox stabilizing the fracture. Incisions were closed by sutures. Day one after surgery the patient was walking under full weight bearing with an ankle brace and was discharged the day after.