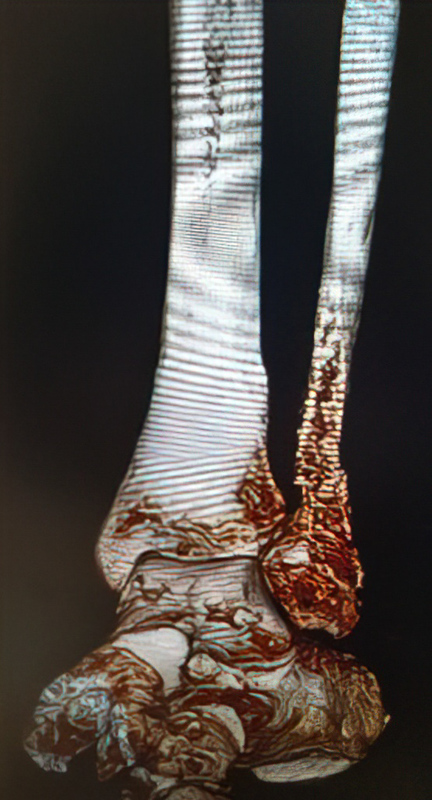
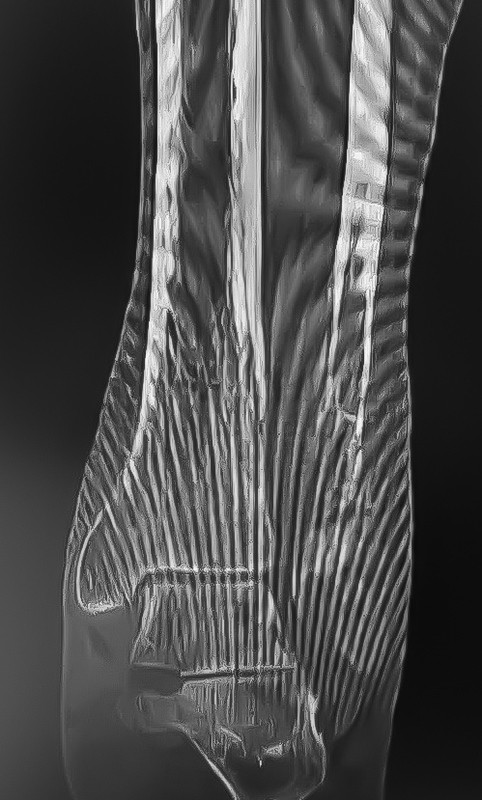
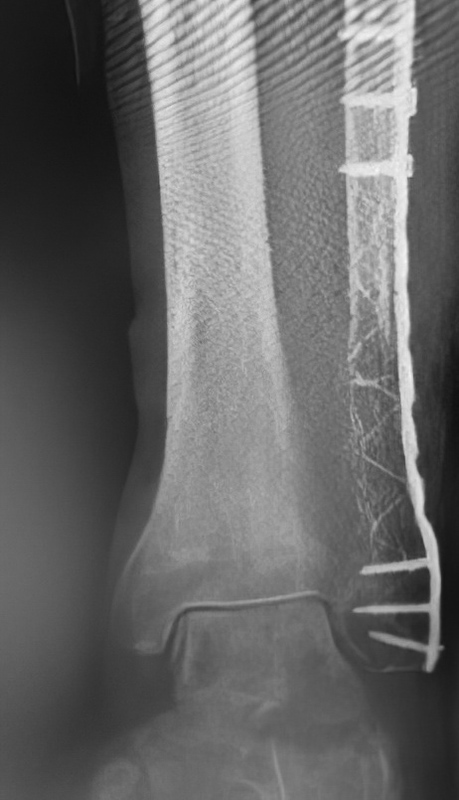
An 86-year-old patient sustained a pathological distal fibula fracture on the left side in the presence of prostate carcinoma. The patient was immobile and relied on a wheelchair for mobility. The fibula was treated by closed reduction by double osteosynthesis using intramedullary stabilization with an IlluminOss 9mm x 160mm implant and a 12-hole plate. Utilizing a small incision in a minimally invasive fashion, the distal fibula was repositioned and the IlluminOss catheter was inserted via the lateral malleolus, filled and expanded with monomer, and then cured with visible light. Post curing, the fibula was augmented with a 12-hole plate and screws for additional stabilization. Post operative X-rays showed proper alignment of the inserted osteosynthesis materials. Peripheral circulation, motor and sensory functions were intact. The patient was discharged after three days of hospitalization with a Vacoped Orthosis, and the patient was allowed to fully weight bear.